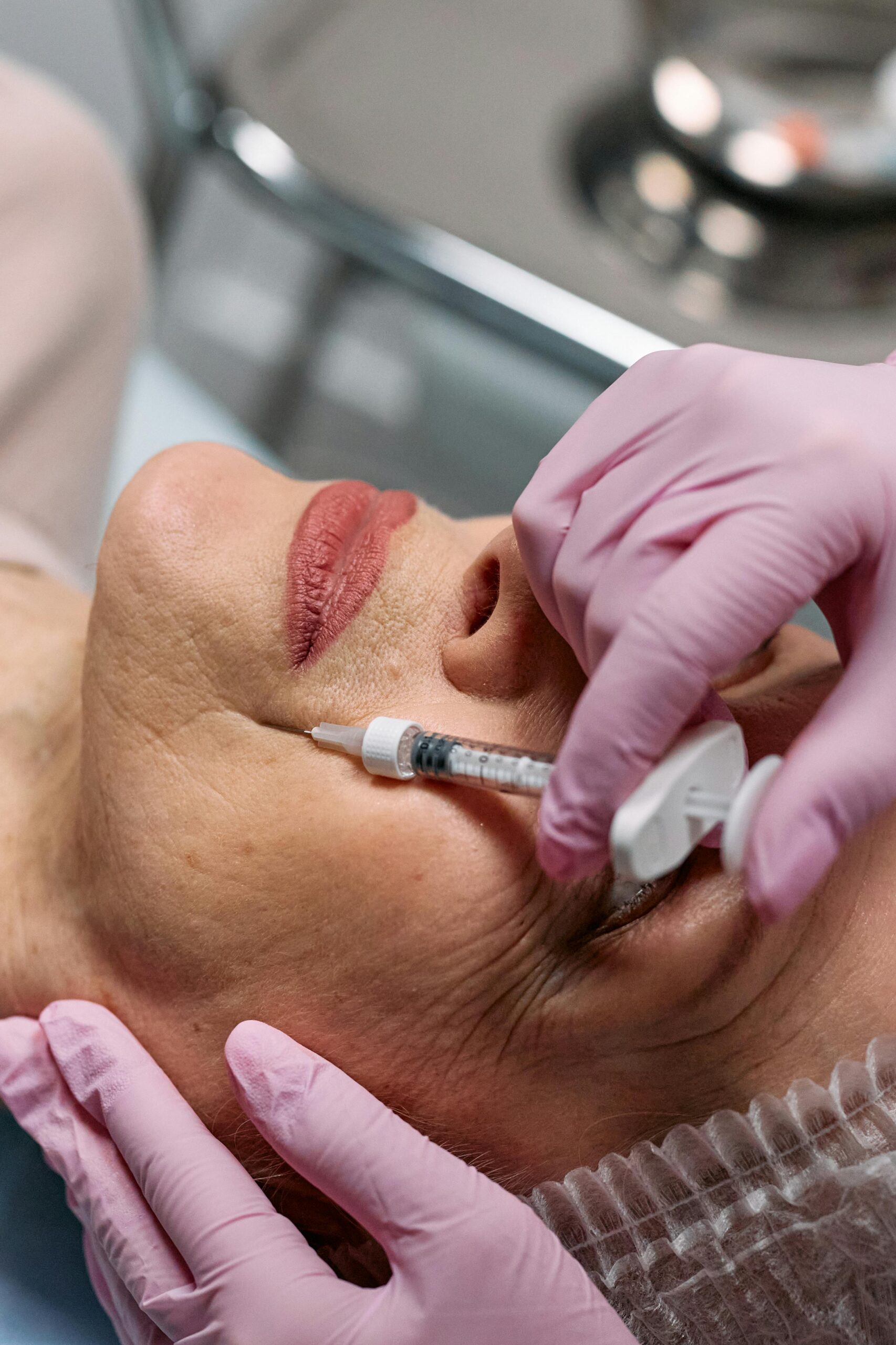
For decades, Botox stood alone as the dominant wrinkle relaxer. It was the brand name everyone recognized, the injectable that defined an entire category. A patient could walk into a provider’s office, ask for Botox, and know exactly what they were getting. Fast forward to 2025, and the landscape looks very different. The United States now has six different FDA-approved neuromodulators for frown lines, with more under review.
The sudden expansion of choices has sparked curiosity. Why do we now have so many competitors in a field that was once a monopoly? Which brands are approved, which ones are not, and which are already used in other parts of the world? Patients are also asking whether these new products really differ from Botox, or if they are simply alternative labels for the same thing.
The answers lie in the convergence of science, global markets, and patient demand. The Botox era is no longer defined by one brand, but by a family of related treatments that offer subtle differences in formulation, longevity, and convenience.
Why the Explosion of Options Now
One reason for the sudden variety is that the demand for minimally invasive cosmetic procedures has grown every year since the early 2000s. According to the American Society of Plastic Surgeons, neuromodulators are consistently the top-requested cosmetic treatment in the country, with millions of injections performed annually. As patients lined up for wrinkle relaxers, the incentive for pharmaceutical companies to create alternatives grew stronger.
Patent timelines also mattered. For many years, Botox was protected by patents that limited competition in the U.S. Once those protections began to expire, other companies were able to bring new versions to market. Each introduced a slightly different formulation or manufacturing process, positioning itself with claims of unique advantages such as faster onset, longer duration, or lower risk of developing resistance.
Globalization also played a role. In Asia and Europe, the neurotoxin market had already become crowded with multiple competing products. South Korean firms, in particular, developed large neurotoxin businesses, exporting products across Asia and Latin America long before those same formulations appeared in the U.S. By the time the FDA granted approvals, some of these products had been used abroad for years.
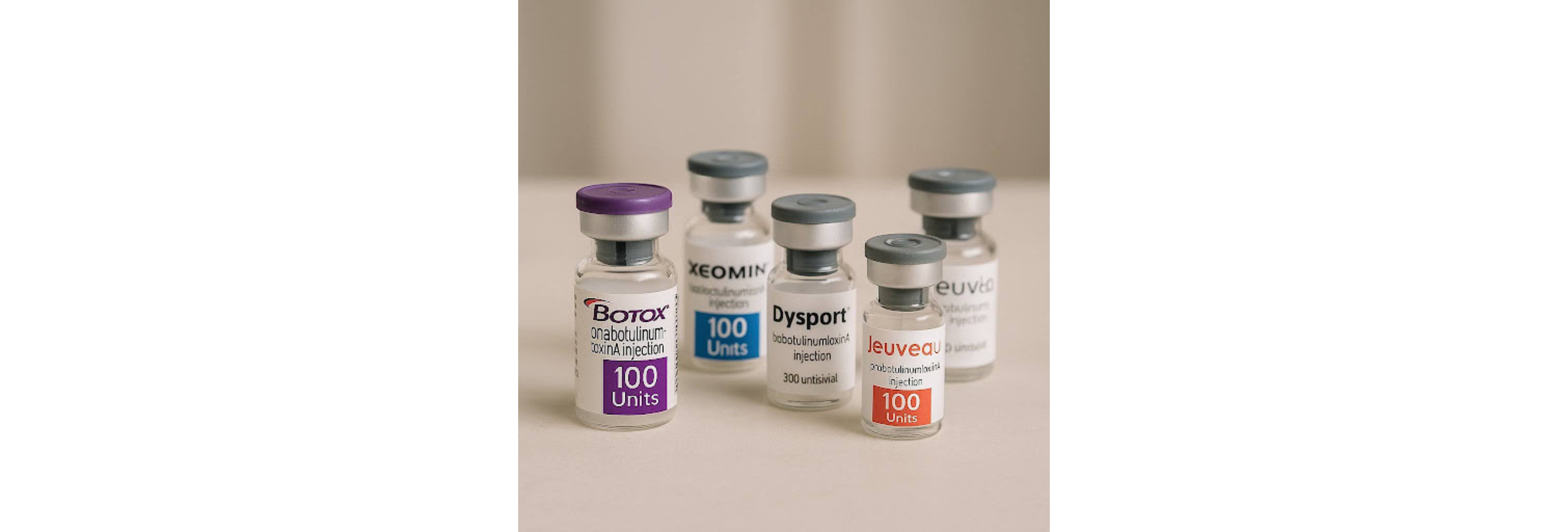
The Six FDA-Approved Neuromodulators
As of 2025, the U.S. market includes six botulinum toxin type A products that are FDA-approved for treating glabellar lines, the frown lines between the eyebrows.
- Botox Cosmetic (onabotulinumtoxinA): The original and most widely known. It set the standard and remains the most requested by name.
- Dysport (abobotulinumtoxinA): Approved in the U.S. in 2009, but widely used in Europe before that. It is often described as having more diffusion, which some injectors find useful for treating larger areas.
- Xeomin (incobotulinumtoxinA): Unique because it is stripped of accessory proteins, making it a “naked” toxin. Some clinicians prefer it for patients who have been receiving injections for many years.
- Jeuveau (prabotulinumtoxinA-xiYx): Launched in the U.S. in 2019 and marketed as “Newtox.” Outside the U.S., it is called Nuceiva in Canada and Europe. It was developed to be more affordable while maintaining results similar to Botox.
- Daxxify (daxibotulinumtoxinA-lanm): Approved in 2022, it uses a stabilizing peptide to extend duration. Clinical trials documented results lasting up to six months or longer, compared to three to four months for traditional options.
- Letybo (letibotulinumtoxinA-wlbg): The newest entrant, approved in 2024. In Asia, this same product was marketed under the name Botulax, where it gained years of real-world experience before entering the U.S. market.
These six represent the current state of FDA-approved neurotoxins, and while they all share the same basic mechanism — blocking the release of acetylcholine at the neuromuscular junction — they differ in formulation, duration, and global history.
The Global Story Behind These Products
One of the most fascinating aspects of this new Botox era is how international the development has been. Products that are new to U.S. patients often have long track records abroad.
- Jeuveau was launched in the U.S. in 2019, but Canadians had already known it for years as Nuceiva. European regulators had also reviewed the product, providing a foundation of safety and efficacy data.
- Letybo is perhaps the clearest example of global cross-pollination. Marketed across Asia as Botulax, it was one of the top-selling toxins in South Korea before receiving FDA approval. Patients in Seoul and other cities had access to it years before Americans did.
- Alluzience, approved in Europe in 2021, became the first ready-to-use liquid botulinum toxin type A. Unlike the powdered formulations that require reconstitution, Alluzience is pre-diluted and designed for consistency. While not FDA-approved in the U.S., its success abroad demonstrates the innovation pipeline shaping the field.
South Korea’s neurotoxin market is particularly advanced, with multiple manufacturers exporting products worldwide. The U.S. is now catching up by allowing some of those formulations to enter its domestic market.
What’s Still Pending
While six products are currently approved, others are advancing through late-stage clinical trials. Galderma has been testing relabotulinumtoxinA in aesthetics, with data suggesting potential refinements in onset and duration. Pharmaceutical companies are also experimenting with liquid formulations, new stabilizers, and even delivery systems that could further reduce the need for frequent injections.
The race is not just about aesthetics. Some companies are pursuing broader therapeutic indications, from migraines to spasticity. These medical applications can help companies establish credibility while also opening new revenue streams.
How These Choices Impact Patients
For patients, the increase in options can feel confusing, but it ultimately brings benefits. Not everyone metabolizes neuromodulators at the same rate. Some people may notice that their results fade faster with one brand than with another. Others may prefer a formulation with a longer track record abroad. Still others may want the convenience of a product that requires fewer visits each year.
More choices also mean more price competition, which could make treatments accessible to a broader population. While the differences between products may be subtle, they matter when multiplied across millions of patients.
The most important factor is not which brand is used, but who is administering it. A skilled injector understands the nuances of each product and tailors treatment based on anatomy, goals, and lifestyle. That is why so many patients prefer to seek care at a trusted Botox clinic, where providers are familiar with all available options and can make evidence-based recommendations.
Safety and Regulation
With more brands available, patients must pay attention to regulation. Only the six products listed earlier are FDA-approved for cosmetic use in the U.S. Other formulations may appear online or in international markets, but without FDA oversight, their safety cannot be guaranteed.
The FDA approval process involves rigorous trials to ensure not just cosmetic effectiveness but also consistent safety outcomes. When patients receive injections from licensed providers using approved products, the risk of adverse events remains low. Complications usually arise when unapproved or counterfeit toxins are used, or when injections are performed by unqualified individuals.
Looking Ahead
The Botox era is entering a new phase. What was once a single brand category is now a diverse landscape shaped by innovation, global collaboration, and consumer demand.
For patients, this means more tailored treatments, potentially longer-lasting results, and broader accessibility. For providers, it means staying current with the details of each formulation and being able to explain the differences clearly. For the industry, it signals the start of even more competition and research into ways to improve convenience and outcomes.
Botox may still be the name most people know, but the reality is that the field has outgrown that singular label. With products like Dysport, Xeomin, Jeuveau, Daxxify, and Letybo joining the stage, and with innovations like Botulax and Alluzience shaping the international market, patients in 2025 are entering a new era of aesthetic choice.
Written in partnership with Tom White